Abstract
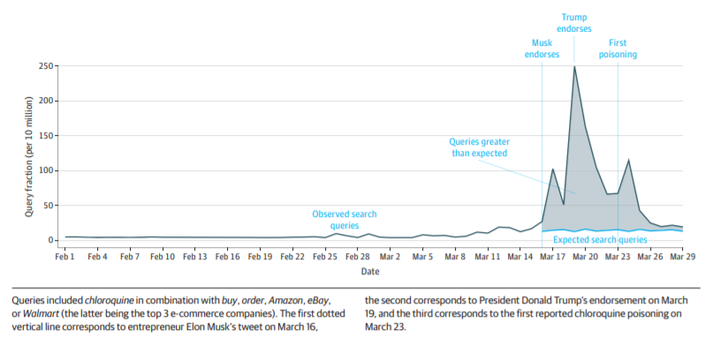
There are no highly effective prescription drug therapies supported by any reliable evidence for the ongoing coronavirus disease 2019 (COVID-19) pandemic of severe acute respiratory syndrome coronavirus 2. However, fears among the public can lead to searches for unproven therapies. Therefore, when several high-profile figures, including entrepreneur Elon Musk and President Donald Trump, endorsed the use of chloroquine, a malarial prophylaxis drug, and hydroxychloroquine (with the antibiotic azithromycin), a lupus and rheumatoid arthritis treatment, to treat COVID-19, it drew massive public attention that could shape individual decision-making. This attention is especially troublesome because chloroquine and hydroxychloroquine (1) are thus far only known to inhibit severe acute respiratory syndrome coronavirus 2 in vitro,1 (2) have potential cardiovascular toxic effects,2 and (3) can be confused with commercially available chloroquine-containing products, such as aquarium cleaner. Poisonings, including 1 fatality, attributed to persons taking chloroquine to prevent or treat COVID-19 without the supervision of a licensed physician have already been reported.3 To better understand the scope of demand for these drugs, we examined internet searches indicative of shopping for chloroquine and hydroxychloroquine.4

Economics & Health Researcher
My research interests include public health, health innovation, and health care.